About HEMA
The Health Economics Methods Advisory (HEMA) group is an independent body of methodologists, policy experts, and researchers. HEMA critically and independently evaluates new methods and processes for health technology assessment (HTA).
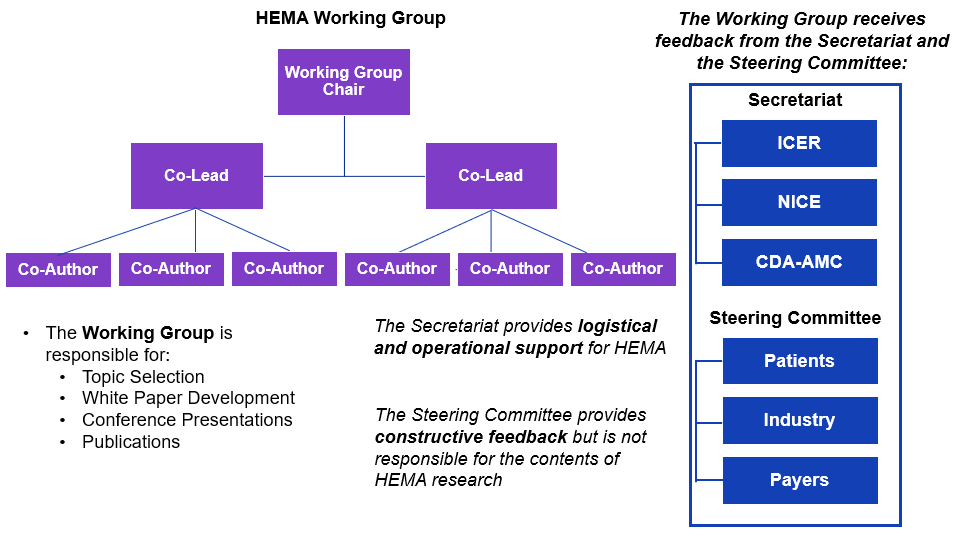
HEMA is convened by Canada’s Drug Agency (CDA-AMC), the U.S.-based Institute for Clinical and Economic Review (ICER), and England’s National Institute for Health and Care Excellence (NICE).
The work is guided by a multi-stakeholder steering committee, comprised of representatives from the patient, payer, and life sciences communities.
HTA is an established practice, conducted by many agencies and research organizations worldwide, to assess and generate evidence to inform decisions about what new medicines and other interventions should be funded from available resources, and at what prices.
Some organizations use economic evaluation as part of HTA, which aims to establish whether the additional benefits generated by (typically new) interventions align with the prices requested, given funding limitations.
The methods of economic evaluation in HTA have developed over time and have always been debated. Some variation in methods across organizations is necessary — it reflects local preferences and priorities. HEMA seeks to evaluate new methods for economic evaluation in HTA as an international community before they are considered for local implementation.
The methods used in HTA work need to be evaluated to ensure the results are in the public’s interest. HEMA aims to be the first global group evaluating HTA methods in a fully independent, credible, and publicly accessible way.
HEMA aims to:
- Examine selected topics — Through the working group and topic specific experts, HEMA will explore topics that include potential benefits, disadvantages, and uncertainties associated with methods, and couple this review with empirical investigation and worked examples where appropriate.
- Provide guidance and recommendations to the HTA community — this guidance may relate to the adoption of novel methods, modifications that might be required, uncertainties in the application of certain methods, and suggestions for further research.
- Coordinate the development of publications — these may include white papers, peer-reviewed articles, workshops, and webinars that focus on the conceptual and empirical applications of alternative methods, assess their applicability and feasibility in HTA settings, and share research and policy perspectives with a broad set of HTA stakeholders.
For any questions related to HEMA’s structure or activities please reach out to Liis Shea, ICER’s Senior Program Director, at [email protected].
To learn more about the HEMA Working Group Principles, click here